Hydrogen Electron Geometry . we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. Linear, trigonal planar, tetrahedral, trigonal. an explanation of the molecular geometry for the h2 (hydrogen gas). all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. The vsepr theory describes five main shapes of simple molecules:
from periodictable.me
Linear, trigonal planar, tetrahedral, trigonal. The vsepr theory describes five main shapes of simple molecules: we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. an explanation of the molecular geometry for the h2 (hydrogen gas).
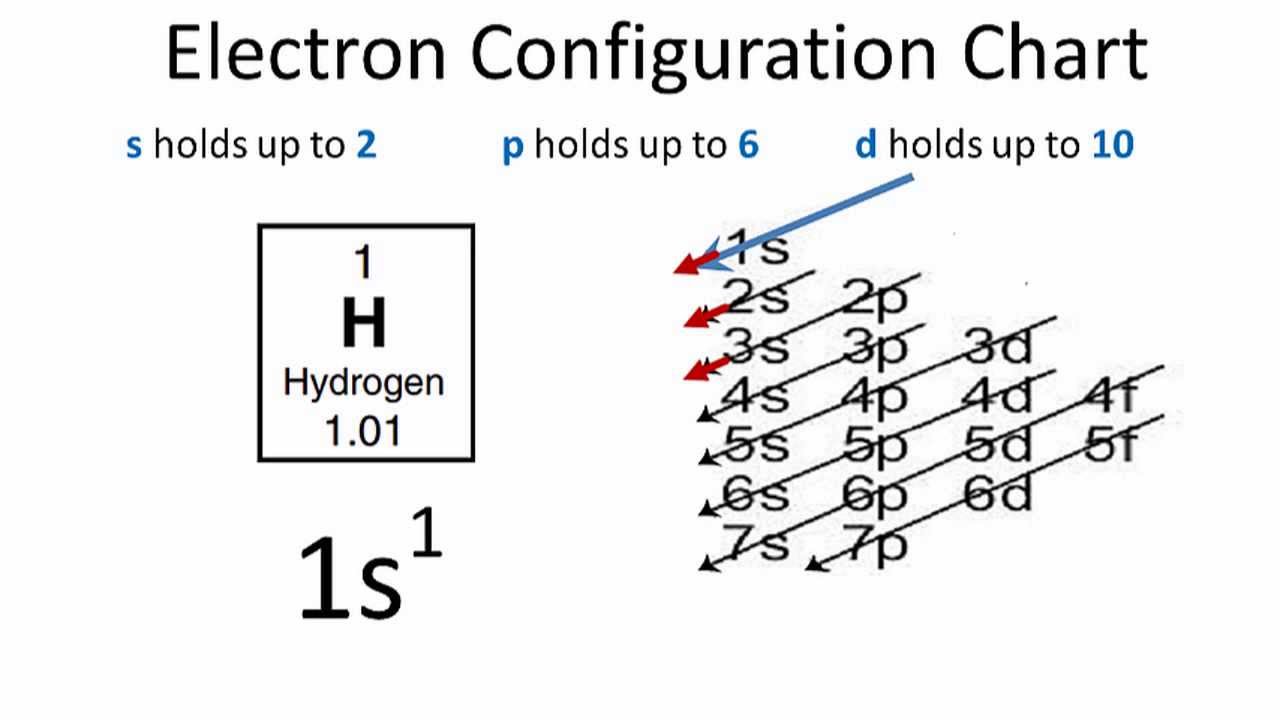
How Can You Find The Hydrogen Electron Configuration (H)
Hydrogen Electron Geometry all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. The vsepr theory describes five main shapes of simple molecules: an explanation of the molecular geometry for the h2 (hydrogen gas). Linear, trigonal planar, tetrahedral, trigonal. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which.
From molecularhydrogeninstitute.org
Dummies Guide To Hydrogen MHI Hydrogen Electron Geometry The vsepr theory describes five main shapes of simple molecules: all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. an explanation of the molecular geometry for the h2 (hydrogen gas). Linear, trigonal planar, tetrahedral, trigonal. we can use the vsepr model to. Hydrogen Electron Geometry.
From homepagetop.com
Hydrogen(H) electron configuration and orbital diagram (2023) Hydrogen Electron Geometry Linear, trigonal planar, tetrahedral, trigonal. The vsepr theory describes five main shapes of simple molecules: an explanation of the molecular geometry for the h2 (hydrogen gas). all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to. Hydrogen Electron Geometry.
From hydrogenatomgirikosa.blogspot.com
Hydrogen Atom Distance Between Proton And Electron In Hydrogen Atom Hydrogen Electron Geometry Linear, trigonal planar, tetrahedral, trigonal. The vsepr theory describes five main shapes of simple molecules: all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. an explanation of the molecular geometry for the h2 (hydrogen gas). we can use the vsepr model to. Hydrogen Electron Geometry.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Hydrogen Electron Geometry all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. The vsepr theory describes five main shapes of simple molecules: an explanation of the molecular geometry for the h2 (hydrogen gas). Linear, trigonal planar, tetrahedral, trigonal. we can use the vsepr model to. Hydrogen Electron Geometry.
From www.reddit.com
Hydrogen Orbitals r/chemistry Hydrogen Electron Geometry Linear, trigonal planar, tetrahedral, trigonal. an explanation of the molecular geometry for the h2 (hydrogen gas). all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by. Hydrogen Electron Geometry.
From chemwiki.ucdavis.edu
The Chemistry of Hydrogen Chemwiki Hydrogen Electron Geometry Linear, trigonal planar, tetrahedral, trigonal. The vsepr theory describes five main shapes of simple molecules: all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. an explanation of the molecular geometry for the h2 (hydrogen gas). we can use the vsepr model to. Hydrogen Electron Geometry.
From chem.libretexts.org
8 The Quantum Hydrogen Atom Chemistry LibreTexts Hydrogen Electron Geometry The vsepr theory describes five main shapes of simple molecules: Linear, trigonal planar, tetrahedral, trigonal. an explanation of the molecular geometry for the h2 (hydrogen gas). all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to. Hydrogen Electron Geometry.
From terrykeirra.blogspot.com
20+ Hydrogen Orbital Diagram TerryKeirra Hydrogen Electron Geometry all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. an explanation of the molecular geometry for the h2 (hydrogen. Hydrogen Electron Geometry.
From periodictable.me
How Can You Find The Hydrogen Electron Configuration (H) Hydrogen Electron Geometry all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. an explanation of the molecular geometry for the h2 (hydrogen gas). The vsepr theory describes five main shapes of simple molecules: Linear, trigonal planar, tetrahedral, trigonal. we can use the vsepr model to. Hydrogen Electron Geometry.
From mungfali.com
Hydrogen Orbitals Hydrogen Electron Geometry The vsepr theory describes five main shapes of simple molecules: we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. Linear, trigonal planar, tetrahedral, trigonal. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons. Hydrogen Electron Geometry.
From www.vectorstock.com
Diagram representation of the element hydrogen Vector Image Hydrogen Electron Geometry we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. Linear, trigonal planar, tetrahedral, trigonal. an explanation of the molecular. Hydrogen Electron Geometry.
From periodictable.me
Valence Electrons in a Hydrogen Atom Archives Dynamic Periodic Table Hydrogen Electron Geometry all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. Linear, trigonal planar, tetrahedral, trigonal. The vsepr theory describes five main shapes of simple molecules: an explanation of the molecular geometry for the h2 (hydrogen gas). we can use the vsepr model to. Hydrogen Electron Geometry.
From brilliant.org
Hydrogen Atom Brilliant Math & Science Wiki Hydrogen Electron Geometry we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. an explanation of the molecular geometry for the h2 (hydrogen. Hydrogen Electron Geometry.
From www.dreamstime.com
Diagram Representation of the Element Hydrogen Stock Vector Hydrogen Electron Geometry Linear, trigonal planar, tetrahedral, trigonal. an explanation of the molecular geometry for the h2 (hydrogen gas). The vsepr theory describes five main shapes of simple molecules: all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to. Hydrogen Electron Geometry.
From www.slideserve.com
PPT Atomic orbital & Hydrogenatom wave function PowerPoint Hydrogen Electron Geometry we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. Linear, trigonal planar, tetrahedral, trigonal. The vsepr theory describes five main shapes of simple molecules: all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons. Hydrogen Electron Geometry.
From www.lancaster.ac.uk
XVII Hydrogen atom‣ Quantum Mechanics — Lecture notes for PHYS223 Hydrogen Electron Geometry Linear, trigonal planar, tetrahedral, trigonal. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which. we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. an explanation of the molecular. Hydrogen Electron Geometry.
From techiescientist.com
H2O Lewis Structure, Molecular Geometry, and Hybridization Hydrogen Electron Geometry an explanation of the molecular geometry for the h2 (hydrogen gas). we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. The vsepr theory describes five main shapes of simple molecules: all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms. Hydrogen Electron Geometry.
From cartridges.esciencelabs.com
Diagram of shapes of molecules, showng bonding pairs, arrangement of Hydrogen Electron Geometry an explanation of the molecular geometry for the h2 (hydrogen gas). Linear, trigonal planar, tetrahedral, trigonal. we can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of. all the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two. Hydrogen Electron Geometry.